Electrochemical CO₂ Splitting
A Path to Sustainable Oxygen and Carbon Production
Exciting news is bubbling up from the world of science, and it’s one that could reshape how we think about carbon dioxide (CO2). Researchers from Nanjing University and Fudan University in China have developed a groundbreaking electrochemical process that splits CO2 directly into carbon and oxygen. This method works under everyday conditions—no extreme temperatures or pressures required—and it could have applications ranging from Earth’s industries to the exploration of Mars.
Nature’s Way vs. New Science
Plants have long been nature’s experts at handling CO2. Through photosynthesis, they convert it into oxygen and glucose, using hydrogen from water as a key intermediary. However, this process isn’t highly efficient, and the oxygen produced comes from water, not CO2 itself. Truly splitting CO2 into its core elements—carbon and oxygen—has been a challenge for scientists, particularly without intense conditions.
That’s where the team, led by Ping He and Haoshen Zhou, steps in. They’ve designed an electrochemical device that accomplishes this feat with remarkable precision. The system includes a gas cathode coated with a nanoscale ruthenium-cobalt (RuCo) cocatalyst and a lithium metal anode. When CO2 enters, it undergoes a two-step reaction with lithium. First, it forms lithium carbonate (Li2CO3), which then breaks down into lithium oxide (Li2O) and solid carbon. An electrocatalytic process then converts the Li2O into lithium ions and oxygen gas (O2). With the optimized RuCo catalyst, the oxygen yield reaches an impressive 98.6%—far exceeding the efficiency of photosynthesis.
A Step Toward a Sustainable Future
This discovery stands out for its adaptability. The team tested it with pure CO2 as well as mixed gases, including simulated flue gas from industrial sources, a CO2-oxygen blend, and a Mars-like atmosphere with just 1% CO2 combined with argon. On Mars, where the atmosphere is predominantly CO2 but pressure is low, this technology could one day generate oxygen for astronauts or habitats. On Earth, it holds promise for applications like underwater oxygen supply, air purification, and waste gas treatment.
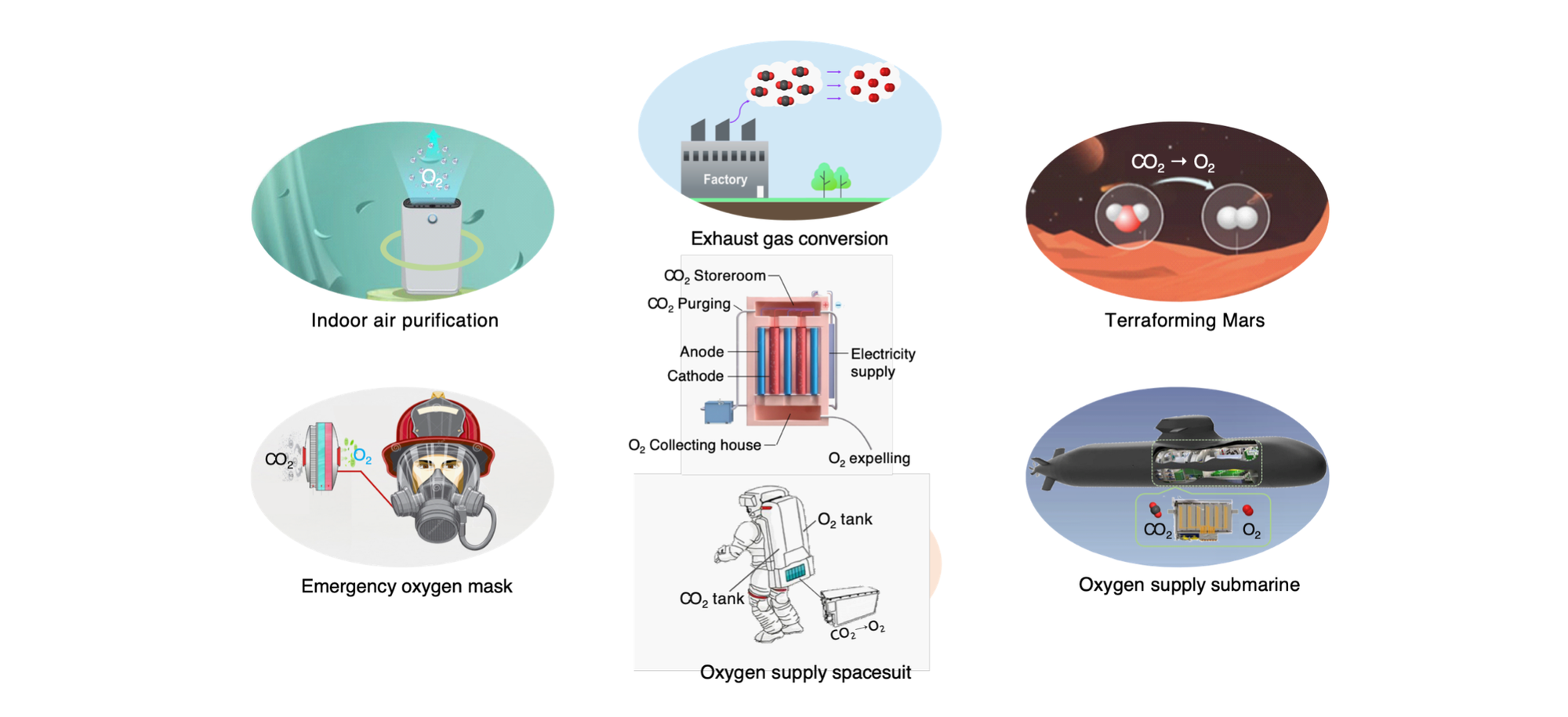
When powered by renewable energy, this process could contribute to carbon neutrality by capturing CO2 and converting it into useful materials. It’s a practical, efficient solution with wide-ranging potential to support industries, space exploration, and environmental goals. For now, this advancement offers a fresh perspective—both literally and figuratively—on tackling CO2 challenges.
