How Jellyfish Regenerate Tentacles
At about the size of a pinkie nail, the jellyfish species Cladonema can regenerate an amputated tentacle in two to three days — but how? Regenerating functional tissue across species, including salamanders and insects, relies on the ability to form a blastema, a clump of undifferentiated cells that can repair damage and grow into the missing appendage. Jellyfish, along with other cnidarians such as corals and sea anemones, exhibit high regeneration abilities, but how they form the critical blastema has remained a mystery until now.

A research team based in Japan has revealed that stem-like proliferative cells — which are actively growing and dividing but not yet differentiating into specific cell types — appear at the site of injury and help form the blastema.
The findings were published in the scientific journal PLOS Biology.
“Importantly, these stem-like proliferative cells in blastema are different from the resident stem cells localized in the tentacle,” said corresponding author Yuichiro Nakajima, lecturer in the Graduate School of Pharmaceutical Sciences at the University of Tokyo. “Repair-specific proliferative cells mainly contribute to the epithelium — the thin outer layer — of the newly formed tentacle.”
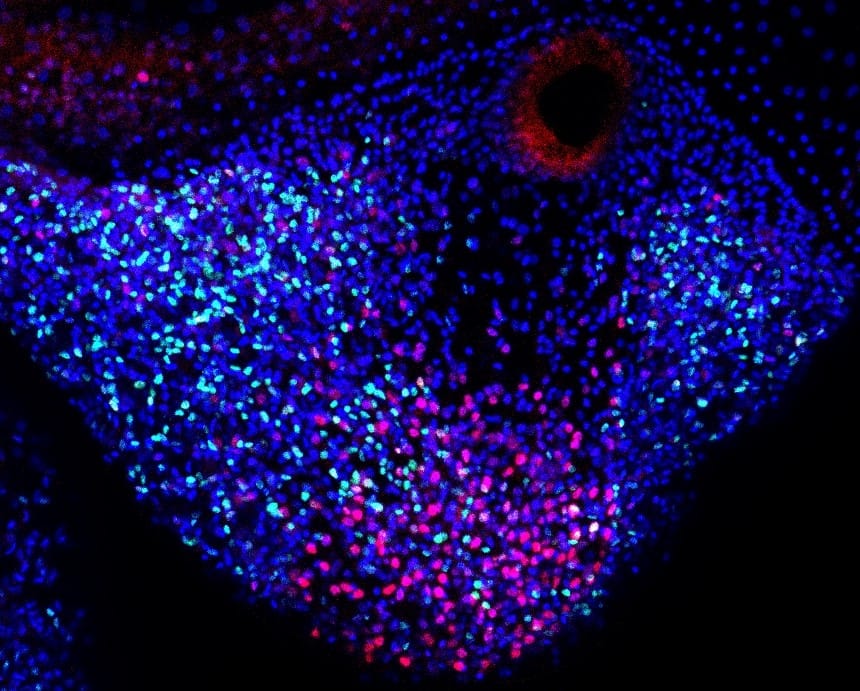
The resident stem cells that exist in and near the tentacle are responsible for generating all cellular lineages during homeostasis and regeneration, meaning they maintain and repair whatever cells are needed during the jellyfish’s lifetime, according to Nakajima. Repair-specific proliferative cells only appear at the time of injury.
“Together, resident stem cells and repair-specific proliferative cells allow rapid regeneration of the functional tentacle within a few days,” Nakajima said, noting that jellyfish use their tentacles to hunt and feed.
This finding informs how researchers understand how blastema formation differs among different animal groups, according to first author Sosuke Fujita, a postdoctoral researcher in the same lab as Nakajima in the Graduate School of Pharmaceutical Sciences.
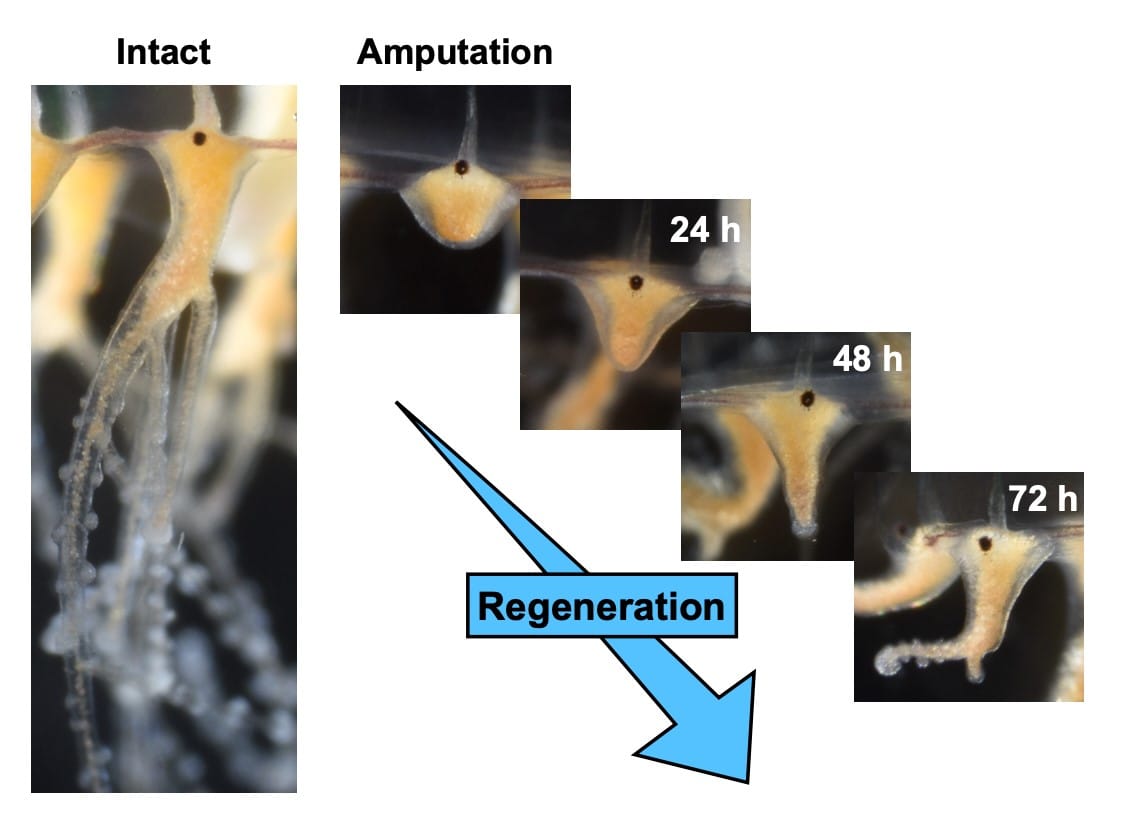
“In this study, our aim was to address the mechanism of blastema formation, using the tentacle of cnidarian jellyfish Cladonema as a regenerative model in non-bilaterians, or animals that do not form bilaterally — or left-right — during embryonic development,” Fujita said, explaining that the work may provide insight from an evolutionary perspective.
Salamanders, for example, are bilaterian animals capable of regenerating limbs. Their limbs contain stem cells restricted to specific cell-type needs, a process that appears to operate similarly to the repair-specific proliferative cells observed in the jellyfish.
“Given that repair-specific proliferative cells are analogues to the restricted stem cells in bilaterian salamander limbs, we can surmise that blastema formation by repair-specific proliferative cells is a common feature independently acquired for complex organ and appendage regeneration during animal evolution,” Fujita said.
The cellular origins of the repair-specific proliferative cells observed in the blastema remain unclear, though, and the researchers say the currently available tools to investigate the origins are too limited to elucidate the source of those cells or to identify other, different stem-like cells.
“It would be essential to introduce genetic tools that allow the tracing of specific cell lineages and the manipulation in Cladonema,” Nakajima said. “Ultimately, understanding blastema formation mechanisms in regenerative animals, including jellyfish, may help us identify cellular and molecular components that improve our own regenerative abilities.”
Written by the University of Tokyo
Images by Sosuke Fujita & The University of Tokyo
