Transforming Industrial CO2 into a Renewable Fuel
Yale scientists have taken a critical next step in creating a scalable process to remove carbon dioxide (CO2) from the air and “recirculate” it as a renewable fuel.
In a new study published in the journal Nature Nanotechnology, Yale chemist Hailiang Wang and his colleagues describe their latest breakthrough in creating methanol — a widely used liquid fuel for internal combustion and other engines — from industrial emissions of CO2, a primary greenhouse gas contributing to climate change.
The process could have far-reaching applications throughout industry.
“This is a new strategy that brings CO2 reduction into methanol to a new level,” said Wang, a professor of chemistry in Yale’s Faculty of Arts and Sciences and lead author of the new study. Wang is also a member of the Yale Energy Sciences Institute and the Yale Center for Natural Carbon Capture.
Transforming CO2 into methanol is a two-step chemical reaction. First, CO2 reacts with a catalyst to become carbon monoxide (CO). The COthen undergoes a catalytic reaction to become methanol.
The most effective previous process — also developed in Wang’s lab — featured a single catalyst made of cobalt tetraaminophthalocyanine molecules supported on carbon nanotubes. But the two reaction steps have a mismatch on this single-site catalyst: the conversion of CO2 to CO is not as efficient or selective, which presents a challenge for scientists trying to devise a robust process that can be scaled up for industrial use.
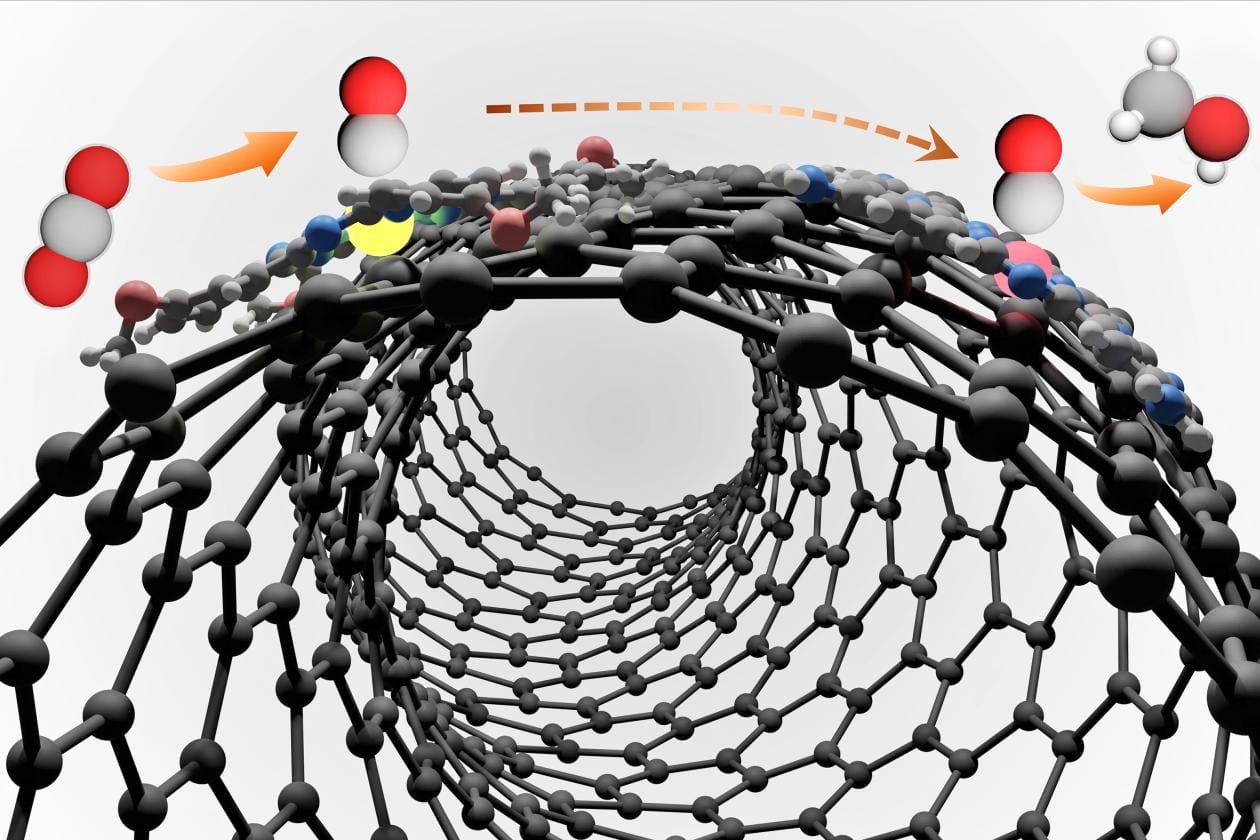
“Having just one type of catalytic site was not optimal for both steps in the reaction,” said Jing Li, a postdoctoral associate in Wang’s lab and first author of the new study. “To avoid this trade-off, we’ve now designed a ‘two-in-one’ catalyst.”
The new process starts with a nickel tetramethoxyphthalocyanine site for the conversion of CO2 into CO. The newly formed CO then migrates onto a cobalt site — catalysis scientists refer to this as “spillover” — to complete the reduction into methanol.
“Our work offers a potentially scalable solution to reduce carbon footprints and accelerate the transition to cleaner energy.” - Conor Rooney
