Nasal Nanodrops
Nasal Drops Deliver Novel Nanoparticles to Combat Brain Tumors Noninvasively
New research from Washington University suggests that nasal drops containing specially designed nanoparticles could provide a noninvasive method to activate the immune system against glioblastoma, a highly aggressive brain cancer. In mouse models, this approach reprogrammed the tumor's immune environment, inhibited growth, and enhanced responses to existing immunotherapies, offering encouraging evidence for less invasive treatment strategies.
The Challenge of Treating Glioblastoma
Glioblastoma, the most common and deadly form of brain cancer, affects about three in 100,000 people annually in the U.S. and progresses rapidly with no curative options available. These tumors, often called "cold tumors," evade the body's natural immune response, making them resistant to standard immunotherapies. Delivering drugs to the brain is particularly difficult due to the blood-brain barrier, a protective shield that blocks many substances. Current experimental treatments targeting the STING pathway—a cellular mechanism that detects foreign DNA and triggers immune activation—require invasive injections directly into the tumor, limiting their practicality for repeated dosing.
A New Approach: Activating the cGAS-STING Pathway with Spherical Nucleic Acids
To address these barriers, researchers developed cGAS-agonistic spherical nucleic acids (SNAs)—tiny structures consisting of gold nanoparticle cores, about 15 nanometers in size (comparable to a virus), densely coated with 45-base-pair double-stranded DNA sequences. These DNA strands are designed to bind to cGAS, an enzyme upstream of STING, prompting the production of cyclic dinucleotides that activate the pathway. This activation releases interferons and cytokines, which help convert immunosuppressive immune cells in the tumor microenvironment into antitumor fighters, such as proinflammatory macrophages.
As Alexander H. Stegh, a professor at Washington University School of Medicine in St. Louis and co-corresponding author of the study, explained: “With this research, we’ve shown that precisely engineered nanostructures, called spherical nucleic acids, can safely and effectively activate powerful immune pathways within the brain. This redefines how cancer immunotherapy can be achieved in otherwise difficult-to-access tumors.”
Unlike traditional STING agonists, which degrade quickly and require invasive delivery, SNAs exhibit improved stability and cellular uptake without needing additional agents.
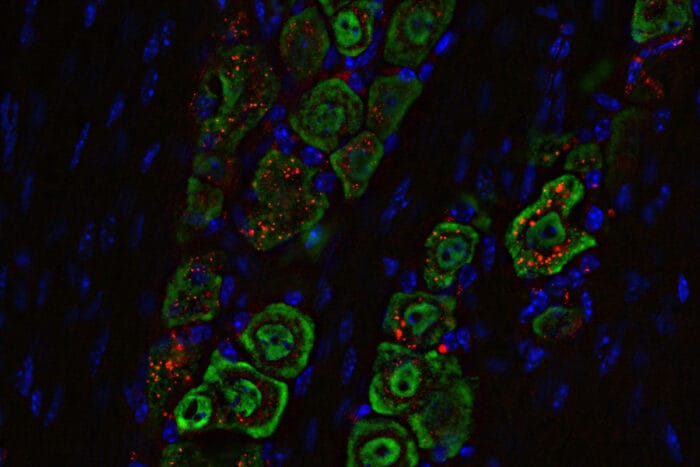
Methodology: Design, Testing, and Delivery
The team, a collaboration between Washington University School of Medicine (led by Stegh) and Northwestern University (led by Chad A. Mirkin, inventor of SNAs), synthesized the SNAs by hybridizing sense and antisense DNA oligonucleotides and attaching them to gold cores. In vitro tests used macrophage cell lines to confirm pathway activation, measuring interferon regulatory factor induction and cytokine production via assays like ELISA and RNA sequencing.
For in vivo studies, researchers administered the SNAs intranasally to mice with syngeneic glioblastoma models (CT-2A and SB28). The nasal route exploits pathways along the olfactory and trigeminal nerves, allowing direct access to the brain without crossing the blood-brain barrier. Fluorescence imaging tracked SNA distribution, showing accumulation in tumor-associated macrophages and trigeminal nerves. Doses were given once or in combination with immune checkpoint inhibitors (anti-PD-L1 and anti-CTLA4), with outcomes monitored via bioluminescence imaging, flow cytometry, and survival analysis.
Akanksha Mahajan, a postdoctoral research associate in Stegh’s lab and first author, noted: “This is the first time that it has been shown that we can increase immune cell activation in glioblastoma tumors when we deliver nanoscale therapeutics from the nose to the brain.”
Results: Immune Reprogramming and Tumor Inhibition
In mouse models, intranasal SNAs inhibited tumor growth more effectively than traditional STING agonists like ADU-S100, extending survival in female mice. The treatment depended on an intact cGAS-STING pathway, as confirmed in STING-deficient mice where no benefits were observed. Flow cytometry revealed an enriched proinflammatory immune microenvironment, with increased effector T cells, activated natural killer cells, and reduced regulatory T cells.
When combined with checkpoint inhibitors, a single dose eradicated tumors in nearly all female mice, inducing long-term immunity against rechallenge. Histopathology and cytokine profiling showed no significant lung toxicity or systemic inflammation, indicating targeted delivery.
Mahajan highlighted the motivation: “We really wanted to minimize patients having to go through that when they are already ill, and I thought that we could use the spherical nucleic acid platforms to deliver these drugs in a noninvasive way.”
Future Implications
These findings indicate that nasal SNA delivery could enhance immunotherapy for glioblastoma by turning "cold" tumors "hot" without invasive procedures.
“This is an approach that offers hope for safer, more effective treatments for glioblastoma and potentially other immune treatment-resistant cancers, and it marks a critical step toward clinical application.” - Alexander Stegh
