Solving the Dolomite Problem
To build mountains from dolomite, a common mineral, it must periodically dissolve. This counter-intuitive lesson could help make new defect-free semiconductors and more.
For 200 years, scientists have failed to grow a common mineral in the laboratory under the conditions believed to have formed it naturally. Now, a team of researchers from the University of Michigan and Hokkaido University in Sapporo, Japan have finally pulled it off, thanks to a new theory developed from atomic simulations.
Their success resolves a long-standing geology mystery called the “Dolomite Problem.” Dolomite—a key mineral in the Dolomite mountains in Italy, Niagara Falls, the White Cliffs of Dover and Utah’s Hoodoos—is very abundant in rocks older than 100 million years, but nearly absent in younger formations.

“If we understand how dolomite grows in nature, we might learn new strategies to promote the crystal growth of modern technological materials,” said Wenhao Sun, the Dow Early Career Professor of Materials Science and Engineering at U-M and the corresponding author of the paper published today in Science.
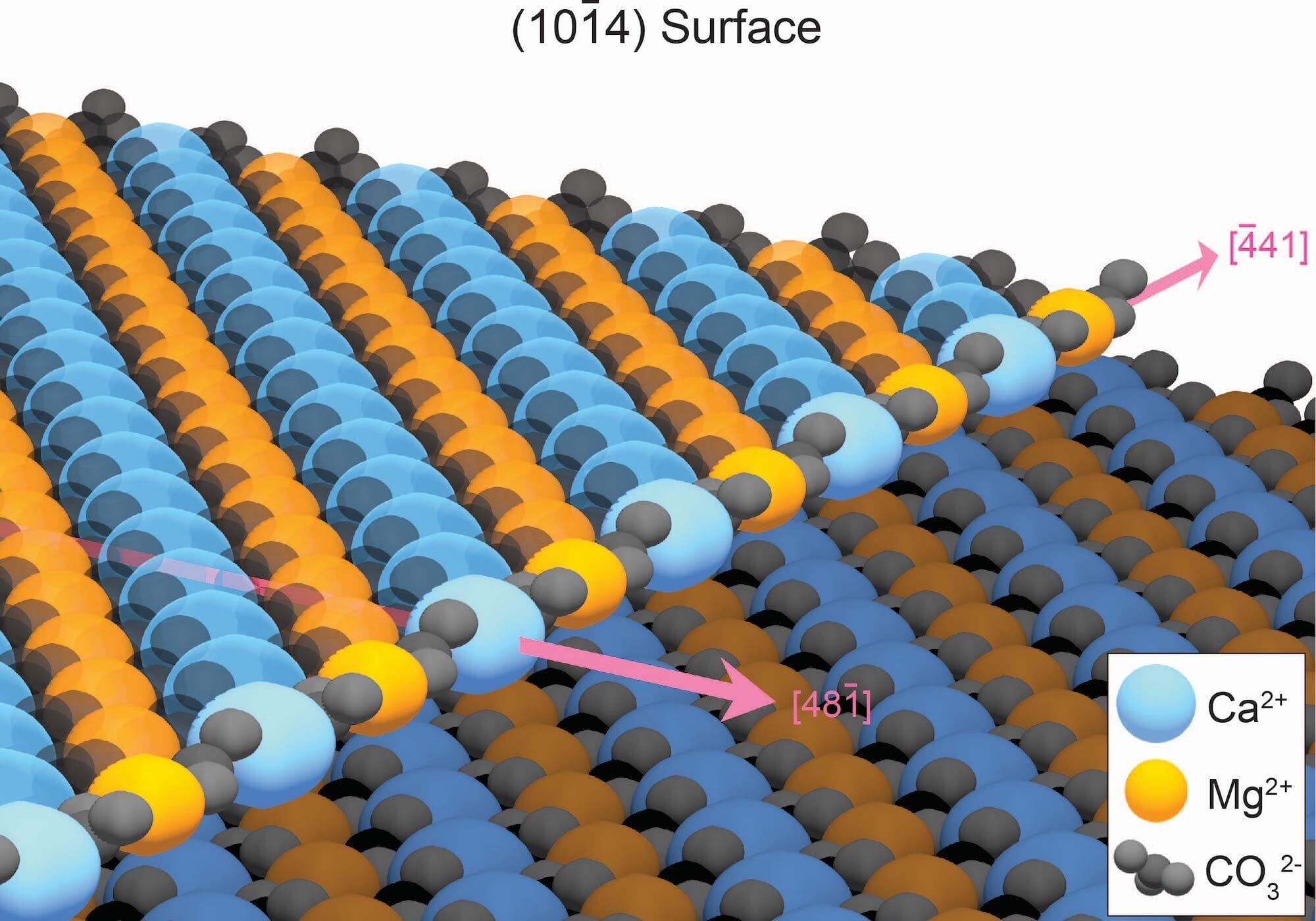
The secret to finally growing dolomite in the lab was removing defects in the mineral structure as it grows. When minerals form in water, atoms usually deposit neatly onto an edge of the growing crystal surface. However, the growth edge of dolomite consists of alternating rows of calcium and magnesium. In water, calcium and magnesium will randomly attach to the growing dolomite crystal, often lodging into the wrong spot and creating defects that prevent additional layers of dolomite from forming. This disorder slows dolomite growth to a crawl, meaning it would take 10 million years to make just one layer of ordered dolomite.
Luckily, these defects aren’t locked in place. Because the disordered atoms are less stable than atoms in the correct position, they are the first to dissolve when the mineral is washed with water. Repeatedly rinsing away these defects—for example, with rain or tidal cycles—allows a dolomite layer to form in only a matter of years. Over geologic time, mountains of dolomite can accumulate.
To simulate dolomite growth accurately, the researchers needed to calculate how strongly or loosely atoms will attach to an existing dolomite surface. The most accurate simulations require the energy of every single interaction between electrons and atoms in the growing crystal. Such exhaustive calculations usually require huge amounts of computing power, but software developed at U-M’s Predictive Structure Materials Science (PRISMS) Centeroffered a shortcut.
“Our software calculates the energy for some atomic arrangements, then extrapolates to predict the energies for other arrangements based on the symmetry of the crystal structure,” said Brian Puchala, one of the software’s lead developers and an associate research scientist in U-M’s Department of Materials Science and Engineering.
That shortcut made it feasible to simulate dolomite growth over geologic timescales.
“Each atomic step would normally take over 5,000 CPU hours on a supercomputer. Now, we can do the same calculation in 2 milliseconds on a desktop,” said Joonsoo Kim, a doctoral student of materials science and engineering and the study’s first author.
The few areas where dolomite forms today intermittently flood and later dry out, which aligns well with Sun and Kim’s theory. But such evidence alone wasn’t enough to be fully convincing. Enter Yuki Kimura, a professor of materials science from Hokkaido University, and Tomoya Yamazaki, a postdoctoral researcher in Kimura’s lab. They tested the new theory with a quirk of transmission electron microscopes.
“Electron microscopes usually use electron beams just to image samples,” Kimura said. “However, the beam can also split water, which makes acid that can cause crystals to dissolve. Usually this is bad for imaging, but in this case, dissolution is exactly what we wanted.”
After placing a tiny dolomite crystal in a solution of calcium and magnesium, Kimura and Yamazaki gently pulsed the electron beam 4,000 times over two hours, dissolving away the defects. After the pulses, dolomite was seen to grow approximately 100 nanometers—around 250,000 times smaller than an inch. Although this was only 300 layers of dolomite, never had more than five layers of dolomite been grown in the lab before.
The lessons learned from the Dolomite Problem can help engineers manufacture higher-quality materials for semiconductors, solar panels, batteries and other tech.
“In the past, crystal growers who wanted to make materials without defects would try to grow them really slowly,” Sun said. “Our theory shows that you can grow defect-free materials quickly, if you periodically dissolve the defects away during growth.”
Written by Derek Smith for University of Michigan News
Images by Joonsoo Kim, Marcin Szczepanski & University of Michigan
